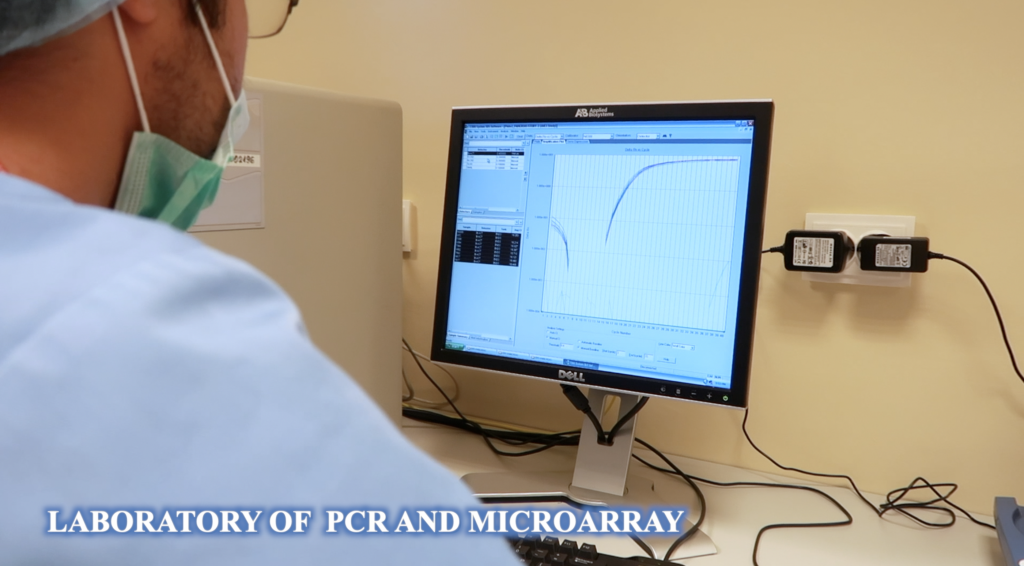
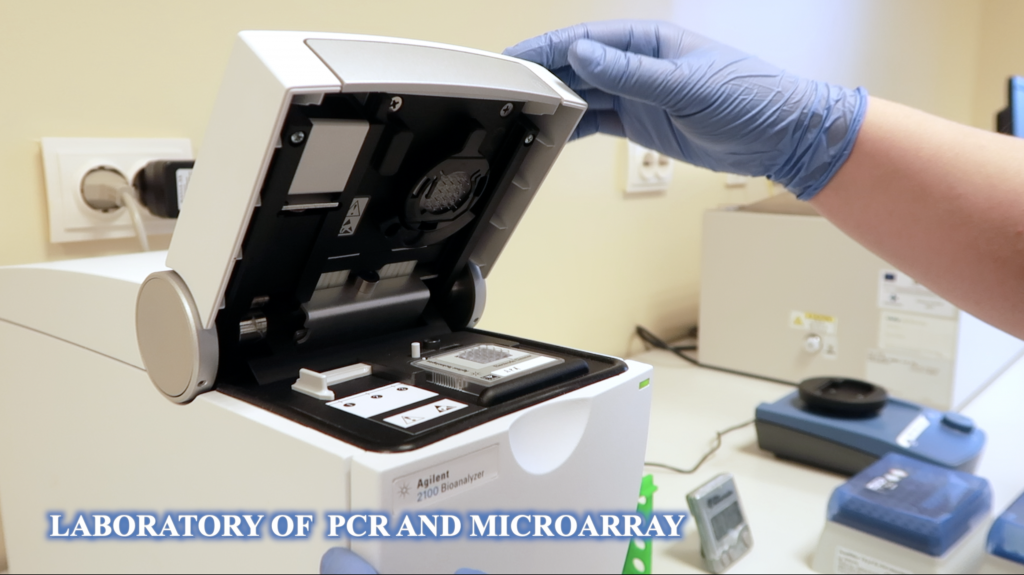
Fundeni Clinical Institute’sBiobank was founded in 2002, together with RNTECH (France), containing biospecimens from patients who underwent surgery in the Center of Digestive Diseases and Liver Transplantation.
Between 2003-2019, specimens (tissue and biological fluids) from over 2500 patients with digestive cancers were included in the biobank ( 14,00 % colon cancer, 14,76 % gastric cancer, 9,35 % rectal cancer, 18,09 % pancreatic cancer, 24,87 % liver cancer, 3,65 % rectosigmoidian junction cancer, 2,09 % metastasis, 13.20 % other cancers). Since December 2018, we have started including specimens (tissue, biological fluids) from patients with gynaecological (20 patients, 0,80%) and breast cancer ( 7 patients, 0,28%).
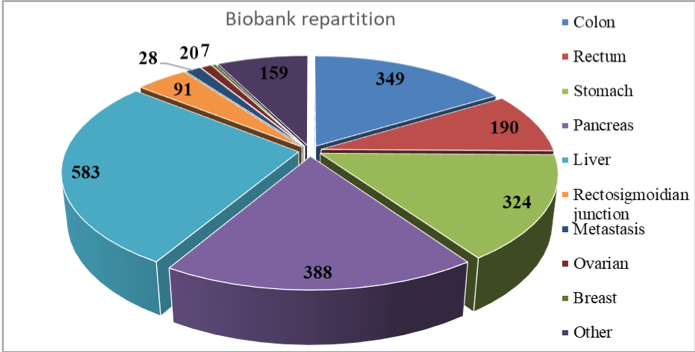
The biological samples are represented by non-tumoral, peritumoral and tumoral tissue, adenoma, metastasis, peritoneal nodules, ascite and when necessary, blood and urine samples. The anonymized database contains relevant information regarding medical history, clinical data, biological and pathological data, as well as information about post-operatory evolution for each patient.
The biological material is collected after obtaining written informed consent, in compliance with international ethical standards and specific European Union legislations (Regulation (EU) 2016/679 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data (General Data Protection Regulation) “, Declaration of Helsikini with regards to ethical principles in medical research that involves human subjects -1964, Parliment and European Councill laws regarding the manipulation and protection of personal data).
Using the biological samples in our biobank, our team has participated in international projects such as FP6 (NEMO: 6 LSH-2005-1.2.2-3, Nano based capsule-Endoscopy with Molecular Imaging and Optical biopsy, Project no:3762) and BBMIR, as well as for our other national projects.