LABORATORY OF BIOLOGICAL SAMPLES PROCESSING
This Laboratory is dedicated to processing various types of biological samples (peripheral blood, tissue).
The biological material is collected after obtaining written informed consent, in compliance with international ethical standards and specific European Union legislations (Regulation (EU) 2016/679 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data (General Data Protection Regulation) “, Declaration of Helsinki with regards to ethical principles in medical research that involves human subjects -1964, Parliament and European Council laws regarding the manipulation and protection of personal data).
Structure:
- Analytical Balance (Sartorius)
- Heraeus Megafuge 8 (ThermoFisher Scientific)
- Hettich® EBA 20 centrifuge (Sigma-Aldrich)
- Mini-spin centrifuge (Eppendorf)
- Thermoblock (FALC)
- PMR-30 rocking shaker (Grant-Bio)
- Refrigerator (Thermo Scientific)

LABORATORY OF NUCLEIC ACID EXTRACTION

Total nucleic acid extraction (total RNA and genomic DNA) is one of the most pivotal steps in molecular biology, being routinely used in many fields of the biological and medical sciences, as this procedure marks a starting point in any molecular scientific experiment.
This laboratory offers an opportunity for genomic DNA and total RNA isolation and analysis since it contains all necessary equipment, such as Maxwell 16 System-benchtop instrument for automated nucleic acid isolation and purification, centrifuge, analytical balance, heating block, stirring hot plate, vortex, spin-down, spectrophotometer and electrophoresis machines. In our lab we have established protocols to isolate high quality total RNA and genomic DNA from tumoral and non-tumoral tissues, plasma, serum, buffy coat, plasma derived exosomes and cell lines.
LABORATORY OF PCR AND MICROARRAY
With the laboratory equipment (Agilent Microarray Scanner, Applied Biosystems 7300 Real Time PCR System, Agilent 2100 Bioanalyzer, 2200 TapeStation Systems) we can perform a variety of targeted gene applications, such as detection of gene expression in cancer and genetic disorders.
Microarray is an important tool for gene expression profiling that can simultaneously evaluate the expression of thousands of genes.
Real-Time PCR Systems offer the flexibility to meet a range of research needs, from basic research and diagnostics to human identification testing.
LABORATORY OF SEQUENCING AND FRAGMENT ANALYSIS


Identifying biomarkers for early diagnosis, monitoring the progression of cancer, screening, and personalized anti-cancer therapy (to achieve the general objectives of translational medicine in oncology).
Our laboratory includes equipment for Sanger sequencing (DNA Sanger Sequencer- Capillary electrophoresis system for nucleic acids (DNA) sequencing) allowing for identification of SNPs involved in different pathologies, as well as equipment for high-throughput next generation sequencing.
The Ion PGM™ System, a reliable sequencing platform that combines simple sample preparation and data analysis solutions with scalable chip output, for ultimate project flexibility. With this system, we can perform a variety of targeted gene sequencing applications, such as variant detection in cancer and genetic disorders.
Furthermore, the NextSeq Series delivers the power of high-throughput sequencing with the speed, simplicity, and affordability of a desktop NGS (next-generation sequencing) system. The fast, integrated, sample-to-results workflow enables many sequencing applications—including transcriptomes, exomes, and targeted panels—in a single run.
With this systems, we can perform a variety of targeted gene sequencing applications, such as variant detection in cancer and genetic disorders.
Infrastructure
- NextSeq 500 Sequencing System (Illumina)
- PGM Ion Torrent™ Sequencing System (Life Technologies)
- DNA Sanger Sequencer- Capillary electrophoresis system for nucleic acids (DNA) sequencing (Beckman Coulter)
- Mini-spin centrifuge (Eppendorf)
- Refrigerator +4/-20°C (Fiocetti)
- Laminary flux hood (Thermo Fisher Scientific)
- Hypex Microsample Incubator (SciGene)
LABORATORY OF PROTEOMICS

Our laboratory includes equipment for Sanger sequencing (DNA Sanger Sequencer- Capillary electrophoresis system for nucleic acids (DNA) sequencing) allowing for identification of SNPs involved in different pathologies, as well as equipment for high-throughput next generation sequencing.
The Ion PGM™ System, a reliable sequencing platform that combines simple sample preparation and data analysis solutions with scalable chip output, for ultimate project flexibility. With this system, we can perform a variety of targeted gene sequencing applications, such as variant detection in cancer and genetic disorders.
Furthermore, the NextSeq Series delivers the power of high-throughput sequencing with the speed, simplicity, and affordability of a desktop NGS (next-generation sequencing) system. The fast, integrated, sample-to-results workflow enables many sequencing applications—including transcriptomes, exomes, and targeted panels—in a single run.
LABORATORY OF BIOBANKING
Goals
Developing the biobank through the inclusion of new digestive tumor cases and improving compatibility of the infrastructure with existing biobanks in Europe.
History
Fundeni Clinical Institute’sBiobank was founded in 2002, together with RNTECH (France), containing biospecimens from patients who underwent surgery in the Center of Digestive Diseases and Liver Transplantation.
Between 2003-2019, specimens (tissue and biological fluids) from over 2140 patients with digestive cancers were included in the biobank ( 16,32 % colon cancer, 15,15 % gastric cancer, 8,88 % rectal cancer, 18,14 % pancreatic cancer, 27,26 % liver cancer, 4,25 % rectosigmoidian junction cancer, 1,31 % metastasis, 8,70 % other cancers). Since December 2018, we have started including specimens (tissue, biological fluids) from patients with gynaecological (20 patients, 0,94%) and breast cancer ( 7 patients, 0,33%).
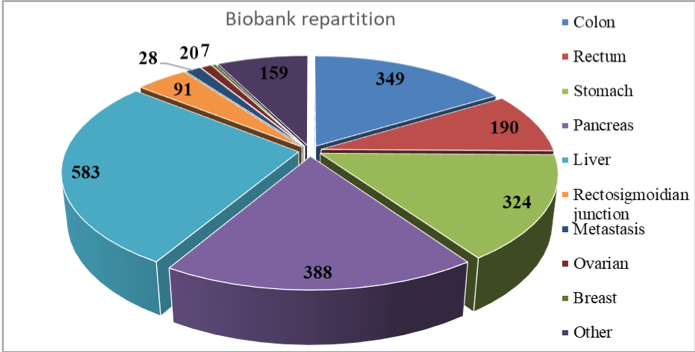
The biological samples are represented by non-tumoral, peritumoral and tumoral tissue, adenoma, metastasis, peritoneal nodules, ascite and when necessary, blood and urine samples. The anonymized database contains relevant information regarding medical history, clinical data, biological and pathological data, as well as information about post-operatory evolution for each patient.
The biological material is collected after obtaining written informed consent, in compliance with international ethical standards and specific European Union legislations (Regulation (EU) 2016/679 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data (General Data Protection Regulation) “, Declaration of Helsikini with regards to ethical principles in medical research that involves human subjects -1964, Parliment and European Councill laws regarding the manipulation and protection of personal data).
Using the biological samples in our biobank, our team has participated in international projects such as FP6 (NEMO: 6 LSH-2005-1.2.2-3, Nano based capsule-Endoscopy with Molecular Imaging and Optical biopsy, Project no:3762) and BBMIR, as well as for our other national projects.
Equipment
- Freezer MDF-U5186S (Sanyo)
- Freezer VIP MDF-U500VX (Sanyo)
- Freezer MDF-U4186S-PE (Panasonic)
- Freezer MDF-U54086S (Sanyo)
- Freezer TSU300V63 (ThermoFisher Scientific)
- Microcentrifuge for 4/6/9 ml tubes with accessories (ThermoFisher Scientific)
- Hettich® EBA 20 centrifuge(Sigma-Aldrich)
- Mini-spin centrifuge (Eppendorf)
- Thermoblock (FALC)
Sample types:

Workflow:
